Background: Gilteritinib, an oral FMS-like tyrosine kinase 3 (FLT3) inhibitor, demonstrated antileukemic responses in patients with FLT3-mutated (FLT3mut+) relapsed/refractory acute myeloid leukemia (AML). We report final results from a phase 1 study of once-daily oral gilteritinib plus intravenous (IV) chemotherapy in patients with newly diagnosed AML.
Methods: This 4-part, open-label, phase 1 study (NCT02236013) assessed the safety/tolerability and antileukemic effects of gilteritinib plus 7+3 induction and high-dose cytarabine consolidation chemotherapy, and as single-agent maintenance therapy in adults with newly diagnosed AML. In part 1, successive cohorts of 3-6 patients received 40-200 mg/d gilteritinib (Days 4-17) and ≤2 cycles of induction (cytarabine 100 mg/m2/d IV, Days 1-7; idarubicin 12 mg/m2/d IV, Days 1-3). In part 2, patients (n=33, of which at least 15 were FLT3mut+) received the recommended 120 mg/d gilteritinib expansion dose and ≤2 cycles of the part 1 induction schedule. In part 3, patients were stratified into 2 cohorts: one receiving treatment from part 2 (n=7) and the other receiving treatment that replaced idarubicin with daunorubicin (90 mg/m2/d IV, Days 1-3; n=7). In part 4, patients (n=12) received the same induction as the part 3/daunorubicin cohort (with a reduction in cycle 2 to daunorubicin 45 mg/m2/d). During consolidation, patients received ≤3 cycles of cytarabine (1.5 g/m2 every 12 hours; Days 1, 3, and 5) and gilteritinib (Days 1-14 for parts 1-3; Days 1-56 for part 4) at the induction dose. Gilteritinib was given once daily in 28-day cycles for up to 26 cycles as maintenance therapy (maintenance phase is still ongoing). Patients achieving composite complete remission (CRc) or partial remission could undergo hematopoietic stem cell transplant (HSCT) and resume maintenance gilteritinib treatment post-HSCT.
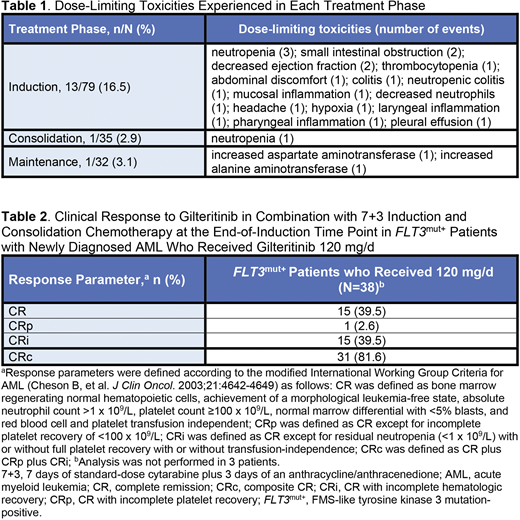
Results: As of 23 June 2020, 80 patients were allocated to treatment (safety analysis set, n=79); median age was 59.0 y (range, 23-77) and most were male (62.0%). Median follow-up for overall survival (OS) was 35.8 mo. Dose-limiting toxicities are provided in Table 1. The maximum tolerated dose was 120 mg/d. Serious treatment-related adverse events (AEs) and AEs leading to discontinuation of gilteritinib occurred in 12.7% (n=10) and 5.1% (n=4) of patients, respectively. One (1.3%) death occurred across all treatment phases. Grade ≥3 nonhematologic AEs (≥10% of patients) were increased alanine aminotransferase (13.9%), pneumonia (13.9%), sepsis (11.4%), and bacteremia (11.4%). At the end-of-induction time point, there were 44 (55.7%) total FLT3mut+ patients across all dose groups and 38 (48.1%) patients who received gilteritinib 120 mg/d. Investigator-reported CRc was achieved by 81.8% of patients across all dose groups (n=36) and 81.6% among patients who received gilteritinib 120 mg/d (n=31; Table 2). Anthracycline choice had no clear impact on CRc rate, although the number of patients in these cohorts was low. In FLT3mut+ patients who achieved CRc in any dose group, median (95% CI) duration of CRc and disease-free survival were 14.1 (4.0-29.9) and 15.3 (9.8-not reached) mo, respectively. Median OS for FLT3mut+ patients has not been reached. The survival probability (95% CI) in all FLT3mut+ patients at weeks 8, 12, 26, 52, and 104 were 97.7% (84.6%-99.7%), 95.3% (82.5%-98.8%), 92.9% (79.6%-97.7%), 83.1% (67.7%-91.5%), and 71.8% (54.6%-83.4%), respectively. In patients with FLT3 internal tandem duplication (ITD)-positive AML achieving CRc, mutational clearance (summed FLT3 ITD signal ratio of ≤10-4 after induction or consolidation) was achieved by 70% (n/N=16/23) of patients receiving a gilteritinib dose of ≥120 mg. HSCT occurred in 30.4% of the total population (n/N=24/79). Analysis of plasma inhibitory activity and pharmacokinetics of gilteritinib will be available at presentation.
Conclusions: Gilteritinib plus induction and consolidation chemotherapy is well tolerated in patients with newly diagnosed AML. Favorable antileukemic responses were observed in FLT3mut+ patients regardless of anthracycline type or gilteritinib administration schedule, with a mutational clearance rate of 70.0%. Based on these results, randomized clinical trials of induction and consolidation chemotherapy plus gilteritinib vs midostaurin in FLT3mut+ AML patients have been initiated.
Pratz:AbbVie: Other: Scientific Advisory Board, Research Funding; Astellas: Other: Scientific Advisory Board, Research Funding; Boston BioMedical: Consultancy; Celgene: Other: Scientific Advisory Board; Agios: Other: Scientific Advisory Board, Research Funding; Jazz Pharmaceutical: Consultancy; Millennium: Research Funding; Daiichi Sankyo: Research Funding. Cherry:Pfizer: Other: Advisory Board; BMS: Other: Advisory Board; Kite: Other: Advisory Board. Altman:PeerView: Consultancy; ASH: Consultancy; Syros: Consultancy; Janssen: Consultancy; Genentech: Research Funding; Amphivena: Research Funding; Amgen: Research Funding; Aprea: Research Funding; ImmunoGen: Research Funding; Celgene: Research Funding; Boehringer Ingelheim: Research Funding; PrIME Oncology: Consultancy; Immune Pharmaceuticals: Consultancy; Novartis: Consultancy; Glycomimetics: Other: Data safety and monitoring committee; Daiichi Sankyo: Other: Advisory Board - no payment but was reimbursed for travel; Kura Oncology: Other: Scientific Advisory Board - no payment accepted, Research Funding; Kartos: Research Funding; AbbVie: Other: advisory board, Research Funding; Fujifilm: Research Funding; BioSight: Other: No payment but was reimbursed for travel , Research Funding; Bristol-Myers Squibb: Consultancy; Astellas: Other: Advisory Board, Speaker (no payment), Steering Committee (no payment), Research Funding; Cancer Expert Now: Consultancy; Agios: Other: advisory board, Research Funding; Theradex: Other: Advisory Board; France Foundation: Consultancy. Cruz:Takeda: Speakers Bureau. Jurcic:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Arog Pharmaceuticals: Research Funding; Astellas: Research Funding; Forma Therapeutics: Research Funding; Genentech: Research Funding; Kura Oncology: Research Funding; PTC Therapeutics: Research Funding; Syros Pharmaceuticals: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Levis:Menarini: Honoraria; Amgen: Honoraria; FujiFilm: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria; Astellas: Honoraria, Research Funding. Lin:Pfizer: Research Funding; Bio-Path Holdings: Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Tolero Pharmaceuticals: Research Funding; Ono Pharmaceutical: Research Funding; Prescient Therapeutics: Research Funding; Incyte: Research Funding; Genetech-Roche: Research Funding; Gilead Sciences: Research Funding; Aptevo: Research Funding; Celyad: Research Funding; Celgene: Research Funding; Astellas Pharma: Research Funding; Mateon Therapeutics: Research Funding; Jazz: Research Funding; Trovagene: Research Funding. Perl:Bayer HealthCare Pharmaceuticals: Research Funding; Syndax: Consultancy, Honoraria; Jazz: Honoraria, Other; Arog Pharmaceuticals Inc: Other: uncompensated consulting, travel costs for meetings; Biomed Valley Discoveries: Research Funding; Agios: Consultancy, Honoraria, Other; FUJIFILM Pharmaceuticals USA, Inc: Research Funding; AbbVie Inc: Consultancy, Honoraria, Other, Research Funding; Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly & Company: Consultancy, Honoraria, Other; Takeda: Honoraria, Other: Travel costs for meeting; Astellas: Consultancy, Honoraria, Other: writing/editorial support, travel costs for meeting presentations related to study, Research Funding; Leukemia & Lymphoma Society, Beat AML: Consultancy; New Link Genetics: Honoraria, Other; Daiichi Sankyo: Consultancy, Honoraria, Other: Writing/editorial support, travel costs for meetings, Research Funding; FORMA Therapeutics: Consultancy, Honoraria, Other; Actinium Pharmaceuticals Inc: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Other, Research Funding. Podoltsev:Arog Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Sunesis Pharmaceuticals: Research Funding; Boehringer Ingelheim: Research Funding; Daiichi Sankyo: Research Funding; Astellas Pharma: Research Funding; CTI biopharma: Consultancy, Honoraria, Research Funding; Bristol-Myers Squib: Consultancy, Honoraria; Genentech: Research Funding; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Samus Therapeutics: Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Blueprint Medicines: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Kartos Therapeutics: Research Funding; AI Therapeutics: Research Funding. Schiller:Abbvie: Research Funding; Actinium: Research Funding; Ariad: Research Funding; Amgen: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Incyte: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Ono Pharma: Consultancy; Kite Pharma: Research Funding; Stemline: Speakers Bureau; Gilead: Speakers Bureau; Celgene: Research Funding, Speakers Bureau; Sanofi: Speakers Bureau; Agios: Consultancy, Research Funding, Speakers Bureau; DeltaFly: Research Funding; Deciphera: Research Funding; Daiichi Sankyo: Research Funding; Cyclacel: Research Funding; Constellation: Research Funding; Celator: Research Funding; Astellas Pharma: Honoraria, Research Funding; Bristol-Myers Squibb: Current equity holder in publicly-traded company, Research Funding; Forma: Research Funding; FujiFilm: Research Funding; Gamida: Research Funding; Genentech-Roche: Research Funding; Geron: Research Funding; Jazz Pharmaceuticals: Research Funding; Karyopharm: Research Funding; Mateon: Research Funding; MedImmune: Research Funding; Onconova: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; Regimmune: Research Funding; Samus: Research Funding; Sangamo: Research Funding; Tolero: Research Funding; Trovagene: Research Funding; Kaiser Permanente: Consultancy; Johnson & Johnson: Current equity holder in publicly-traded company. Hill:Targeted Molecular Diagnostics: Patents & Royalties: US7862995; Astellas: Current Employment; Ligacept, LLC: Current equity holder in publicly-traded company, Patents & Royalties: US9051388, US9683222. James:Astellas: Current Employment. Lu:Astellas: Current Employment. Tiu:Astellas Pharma Global Development: Current Employment; Eli Lilly & Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months.
New Indication
Author notes
Asterisk with author names denotes non-ASH members.